The radiocarbon method, developed more than 60 years ago and awarded the Nobel Prize, was originally used to determine the age of archaeological and geological objects, but soon its scope expanded significantly. The method has proven its versatility and continues to be applied with great success in science, technology, medicine and other areas of human activity.
More ...
Hide
Historical reference
The radiocarbon method has a significant impact on the development of various fields of science - from nuclear physics to forensic science, but primarily geology and archeology. In March 1949, an article was published, which substantiated the principle of operation of this method. Its authors - scientists from the University of Chicago (USA) Willard F. Libby, Ernst S. Anderson and James R. Arnold - have shown that they can determine the age of geological or historical events that took place not only hundreds and the first thousand years ago, but also up to 40-50 thousand years ago. At the same time, the proposed method had a sufficiently high accuracy and was completely independent of other technologies used at that time in the sciences. It can be said without exaggeration that the radiocarbon method has made a real revolution in the concept of time in scientific knowledge.
The first radiocarbon dates were obtained by Willard Libby in 1949 at the University of Chicago. It should be emphasized that this became possible thanks to the many years of efforts of a rather large team working under the leadership of W. Libby in various fields of science. Thus, the possibility of converting atmospheric nitrogen when bombarded with neutrons into the carbon isotope 14C was predicted theoretically back in the mid-30s of the 20th century. In laboratory conditions, such a reaction was carried out in 1940, at about the same time neutrons were recorded in the upper atmosphere, produced under the influence of cosmic radiation. Thus, one of the basic principles of radiocarbon dating - 14C is formed in the upper atmosphere under the influence of cosmic rays - was formulated by the beginning of the 1940s. Further work in this direction was interrupted by the Second World War, during which W. Libby participated in the Manhattan Project. After the war, the half-life of radiocarbon was measured and methods were developed for determining its activity in plant and animal tissues. The fact is that the ratio of 14C to other isotopes of carbon in the atmosphere is only one in 1012 atoms. Accordingly, the activity due to radiocarbon is also very low.
Immediately after the first works of W.F. Libby and his colleagues, the American Anthropological Association and the US Geological Society created a special commission to evaluate the first results of radiocarbon dating, which in 1951 came to the conclusion about the reliability of the data obtained and their compliance with the existing scientific paradigm. The scientific community enthusiastically embraced the new research approach and began to actively use it in studying the past of the Earth and humanity; for many years the method became the leading one in determining the age of certain objects. Since the mid-1950s, the radiocarbon method has spread throughout the world.
Calibration of 14C dates became one of the directions of radiocarbon research, important for all sciences, in the 1960s – 2000s. The need for calibration is due to the fact that the amount of 14C isotope in the atmosphere, hydrosphere and biosphere did not remain constant (as WF Libby and his colleagues initially believed), but changed under the influence of a number of external conditions, the main of which is fluctuations in the recent geological past. activity of cosmic rays producing radiocarbon. Therefore, the relationship between 14C and calendar age is not linear. The influence of this factor, which complicates the translation of the radiocarbon age into astronomical (calendar) dates, has now been overcome for the time interval from the present day to 20,000 years ago; work is being successfully carried out on the preparation of schedules for converting 14C-dates into calendar dates up to the sensitivity limit of the radiocarbon method (about 45,000-50,000 14C years).
Radiocarbon Basics
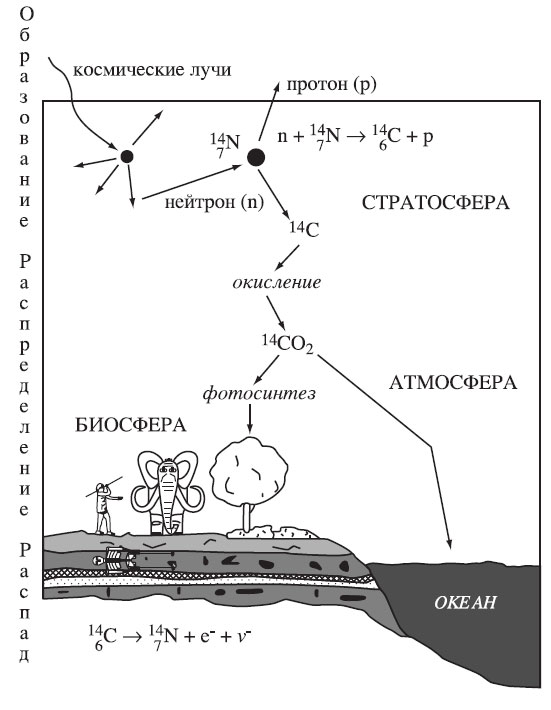 In the natural environment of the Earth, the chemical element carbon consists of three isotopes: two stable - 12C and 13C and one radioactive - 14C, or radiocarbon. The 14C isotope is constantly formed in the Earth's stratosphere as a result of the bombardment of nitrogen atoms with neutrons that are part of cosmic rays. For several years, the "newborn" 14C, along with the stable isotopes 12C and 13C, gets into the Earth's carbon cycle in the atmosphere, biosphere and hydrosphere. While the body is in a state of metabolism with its environment (for example, a tree receives carbon in the form of carbon dioxide from the atmosphere as a result of photosynthesis), the 14C content in it remains constant and is in equilibrium with the concentration of this isotope in the atmosphere. When the organism dies off, the exchange of carbon with the external environment stops; the content of the radioactive isotope begins to decrease, since there is no longer an influx of "fresh" 14C from the outside. The radioactive decay of any element occurs at a constant rate, which is very precisely determined. So, for the 14C isotope, the half-life is about 5730 years. Therefore, knowing the initial amount of 14C in the body in relation to the stable isotopes 12C and 13C in a state of equilibrium (when the body is alive) and the 14C content in the fossil remains, it is possible to establish how much time has passed since the death of the carbon-containing substance.
In the natural environment of the Earth, the chemical element carbon consists of three isotopes: two stable - 12C and 13C and one radioactive - 14C, or radiocarbon. The 14C isotope is constantly formed in the Earth's stratosphere as a result of the bombardment of nitrogen atoms with neutrons that are part of cosmic rays. For several years, the "newborn" 14C, along with the stable isotopes 12C and 13C, gets into the Earth's carbon cycle in the atmosphere, biosphere and hydrosphere. While the body is in a state of metabolism with its environment (for example, a tree receives carbon in the form of carbon dioxide from the atmosphere as a result of photosynthesis), the 14C content in it remains constant and is in equilibrium with the concentration of this isotope in the atmosphere. When the organism dies off, the exchange of carbon with the external environment stops; the content of the radioactive isotope begins to decrease, since there is no longer an influx of "fresh" 14C from the outside. The radioactive decay of any element occurs at a constant rate, which is very precisely determined. So, for the 14C isotope, the half-life is about 5730 years. Therefore, knowing the initial amount of 14C in the body in relation to the stable isotopes 12C and 13C in a state of equilibrium (when the body is alive) and the 14C content in the fossil remains, it is possible to establish how much time has passed since the death of the carbon-containing substance.
In other words, by finding in nature and in the settlements of ancient humans the remains of plants and animals, as well as some other substances containing carbon, it is possible using the radiocarbon method to determine how much time has passed since the end of the life of the organism, that is, to establish the age of these objects. And this, in turn, means that one can answer the eternal question of geologists and archaeologists: how long has this organism or an ancient settlement existed?
It is known that the chemical element carbon is a part of almost all living matter, as well as many substances from the category of inanimate (that is, created without the participation of living organisms). Thus, the radiocarbon method is truly universal. With its help, the age of a number of objects is determined, which can be conditionally divided into the following groups: "geological" - carbonate sediments of the oceans and freshwater bodies, ice cores, meteorites; "biological" - wood and charcoal, seeds, fruits and twigs of plants, peat, soil humus, pollen grains, remains of insects and fish, bones, horns, tusks, teeth, hair, skin and skins of vertebrates and humans, coprolites; "anthropogenic" - burnt bones, ceramics, screeching metal, burnt food remains, traces of blood on ancient tools, fabrics, papyrus, parchment and paper. In some cases, for example, to study fluctuations in 14C content depending on solar activity, its activity is measured in such "exotic" objects as wines, whiskey and cognacs.
Application of the radiocarbon method
Archeology and Quaternary geology have been and remain the main areas of use of the radiocarbon method. In archeology, the use of an independent method of determining the age was truly revolutionary and significantly changed the existing archaeological concepts. It is currently impossible to carry out serious archaeological work without the use of radiocarbon dating. Now, along with the analysis of "routine" objects, which can be attributed to wood, charcoal and bones, the age of such unsuitable materials in the recent past as individual seeds and fruits of plants, textiles, fatty acids (lipids) in ancient ceramics and the ceramics itself, the remains of blood on stone tools, rock paintings.
Known dates
The most famous is the Shroud of Turin. It is widely known that dating was carried out on accelerating mass spectrometers in three famous laboratories (in Oxford, Zurich and Tusson), which received similar results: with a probability of 95%, the material of the shroud was made in the interval from 1260 to 1390. It is much less known that, along with samples of the shroud, three other tissue samples were analyzed in laboratories (the cloak of Louis IX, made between 1240 and 1270, a shroud from an Egyptian burial, woven around 1100, and a cloth that wrapped an Egyptian mummy, dating from about 200 ). In all three cases, the dating obtained in the laboratories coincided with the original data.
A study of the remains of the "Noah's Ark" on Mount Ararat showed an age of only 1200-1400 years, and not at least 5000 years according to biblical chronology.
Among the most famous artifacts dating back to radiocarbon dating are the Qumran scrolls and several early Qur'anic manuscripts. In all these cases, dating confirmed the authenticity of the documents.
The Tyrolean Ice Man or Ötzi, a mummy discovered in a glacier in northern Italy in 1991, became well known. The ideal preservation of the mummy has made it possible to carry out many studies related to anthropological and historical issues. Radiocarbon dating has shown that Oetzi lived between 3300 and 3000 BC. e. Note that in the permafrost of Siberia and Alaska, several almost intact mummies of mammoths, bison, horses, and even one gopher were found. All these findings immediately became the objects of a comprehensive study of zoologists, botanists, geneticists and, of course, specialists in the field of radiocarbon dating.
It is important to give an example of another kind, when the subject of dating is not a separate artifact or a unique find, but a large-scale event. Such was the eruption of a volcano on the island of Terra or Santorini. It is possible that the echoes of this eruption got into the Bible under the guise of Egyptian executions. Traditionally, this event dates back to 1500 BC. e. However, the analysis of numerous (more than 150) radiocarbon dating of various materials from the eastern Mediterranean associated with the traces of the eruption and the tsunami caused by it, including the olive branch buried directly by ash, pushes the date back more than a hundred years ago, to the end of the 17th century BC. e.
Main limitations of the method
The main limitations of the method are due to the origin of the dated materials and the time range in which it operates. Any radiometric age determination method works like a clock. Imagine that you wind up a mechanical watch, from this moment, until the winding ends, it will show the correct time. In the case of radiocarbon dating, we need a material that exchanges carbon with the environment for some time. It is necessary that after a certain moment this exchange stops, then the natural decay of 14C will be a measure of the time elapsed since the exchange was stopped. All living organisms are ideally suited for these conditions: until the moment of death, the concentration of radiocarbon in them corresponds to the concentration of this isotope in the atmosphere. Then the exchange stops and the clock starts working. Thus, radiocarbon dating determines the time of death of an organism, and this is also one of the limitations of the method - imagine an artifact made from something that has a plant (for example, tissue or wood) or animal (say, bone) origin and passed from generation to generation. Radiocarbon dating will show the time of death of a plant or animal, and not the time of construction of the monument in which this artifact was found. This feature of the method is well understood by art experts, for whom the dating of a board or canvas does not serve as a final confirmation of the authenticity of an icon or painting.
Apart from the objects of the organic world, only a few rather exotic materials can be used for radiocarbon dating. For example, quicklime (CaO) was widely used in the construction of houses and fortresses. Combining with water and atmospheric carbon dioxide, it turns into calcium carbonate, firmly holding the stones together. In this case, the exchange with atmospheric carbon dioxide stops after the mortar has hardened, which makes it possible to determine the time of construction of this structure.
As for the time range, the "factory" of the radiometric clock ends after 13 half-lives of this isotope, which in the case of the radiocarbon method is about 70 thousand years. It should be noted that no matter how the 14C content is measured, for samples less than 300 years old, the measurement uncertainty will be quite large, therefore, in such cases, this method is usually not used. The exceptions are samples from the second half of the 20th century. As a result of ground-based nuclear tests, the 14C content in the atmosphere has almost doubled. This makes it possible to distinguish, say, a wine or whiskey from 1963 from younger counterparts.
Material suitable for radiocarbon analysis
The methodological basis of radiocarbon dating is that 14C is formed in the upper atmosphere and is immediately included in the composition of carbon dioxide molecules. Therefore, it is possible to determine the age of only those objects that are somehow associated with atmospheric CO2. First of all, these are any living organisms. Plants and many bacteria in the process of photosynthesis build complex organic molecules from atmospheric carbon dioxide, which serve as the basis of life for all other organisms. This means that it is possible to date the actual carbon of any living creature. However, it should be borne in mind that over the millennia that have passed since the burial of this sample of once living matter, all of its carbon could be replaced by other elements or completely decomposed. This often happens with ancient objects. It is clear that dating is impossible in such cases.
Biogenic carbon is stored in lake and bog sediments, in caves and river sediments, in archaeological sites, even in desert sunburn - a thin film covering the surface of stones. The age of some objects in the inorganic world can also be determined by radiocarbon dating.
Ice samples from Antarctic and Greenland glaciers contain air bubbles trapped when the water freezes. This air corresponds to the composition of the atmosphere at the time of ice formation. Various studies of gases from such bubbles are now being actively pursued. They have already produced many interesting results. We also mention here that the age of some of the samples was successfully determined by dating carbon dioxide. In the same way, that is, by measuring the activity of radiocarbon CO2, it is possible to determine the age of ground and ocean waters. This made it possible to reconstruct changes in the amount of precipitation in the Sahara, to study the rate of exchange of carbon dioxide between the atmosphere and the ocean, etc.
The carbon content in various biological objects varies greatly. For example, dating of wood is carried out mainly on cellulose, and bone residues on collagen. In this case, cellulose makes up about 40% of the mass of wood, while collagen makes up about 20% of the mass of bones. Secondly, the safety of the material depends on climatic factors and the conditions of the burial of the object. The remains of plants and animals found in permafrost or in water-saturated sediments contain much more carbon than similar objects from, say, the soils of central Russia. Finally, each laboratory has its own requirements for the amount of material to be dated, since the instruments used are different.
Based on interviews with Ya.V. Kuzmin and B.F.Khasanov.
Examination of antiques and cultural property
| Service (for 1 sample) | Deadlines | Price without VAT* |
| Dating, age determination | ||
| Wood age determination (LSC-A 14C) |
up to 45 days | 431 USD |
| Determining the age of bones, ceramics, soil (LSC-B 14C) |
up to 45 days | 543 USD |
| Age determination by LSC-A (CO2 absorption) | ||
| inorganics (secondary carbonates, shells, atmospheric CO2) | up to 90 days | 619 USD |
| inorganics with additional processing (dissolved inorganic carbon in water) | up to 90 days | 805 USD |
| organics (wood, coal, peat, soil/sediment, grains, leaves, moss, seeds) | up to 90 days | 694 USD |
| organic with extra processing (bones, teeth, household waste, tires) | up to 90 days | 1177 USD |
| Age determination by LSC-B method (benzene synthesis) | ||
| inorganics (secondary carbonates, shells, atmospheric CO2) | up to 90 days | 880 USD |
| inorganics with additional processing (dissolved inorganic carbon in water) | up to 90 days | 1103 USD |
| organics (wood, coal, peat, soil/sediment, grains, leaves, moss, seeds) | up to 90 days | 1028 USD |
| organic with extra processing (bones, teeth, household waste, tires) | up to 90 days | 1214 USD |
| AMS age determination (14C + d13C) | ||
| inorganics without processing | up to 90 days | 1586 USD |
| inorganics with additional processing (cremated bones and similar specimens) | up to 90 days | 1586 USD |
| organic (paper, parchment, fabric, canvas) | up to 90 days | 1698 USD |
| organic with extra processing | up to 90 days | 1884 USD |
The prices are approved by the director of LLC "In Consulting" 13.02.2026. Deadlines are indicated in working days
For a free consultation, you can use On-line consultation, or call us or write to the messengers.For information about the cost of services go to Tariffs or place Application for Services.








































